
EU-Netzwerk
Der Schutz vor Arzneimittelfälschungen ist eine internationale Aufgabe. Deshalb arbeiten die Länder der EU und des EWR im Rahmen des Europäischen Fälschungsschutzsystems EMVS zusammen. Dieses besteht aus den einzelnen nationalen Systemen (z.B. dem securPharm-System in Deutschland) und einem zentralen Datenrouter (dem EU-Hub). Über den EU-Hub tauschen die nationalen Systeme Daten aus. Der EU-Hub dient auch als Zugangspunkt für pharmazeutische Unternehmer und wird von der European Medicines Verification Organisation (EMVO) betrieben.
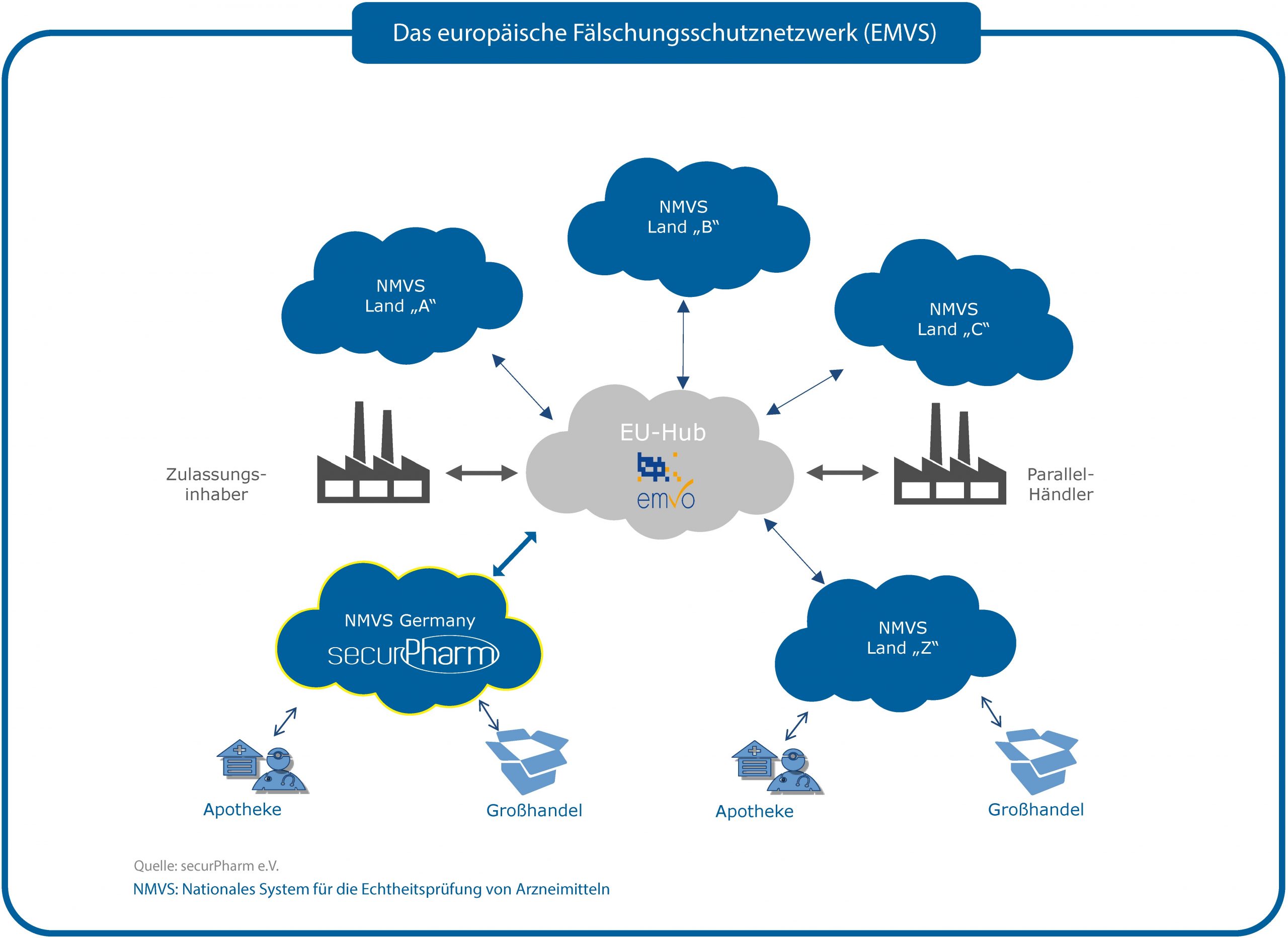
Der EU-Hub ermöglicht beispielsweise, dass jede von der Fälschungsschutzrichtlinie betroffene Packung in jeder Apotheke in der EU überprüft werden kann.
Ablauf der länderübreifenden Verifikation
Dazu scannt die Apotheke einfach den Data Matrix Code auf der Packung. Dabei sendet sie eine Anfrage an ihr nationales System (z.B. das securPharm-System). Kennt das nationale System den Produktcode und die Chargenbezeichnung nicht, leitet es die Anfrage an den EU-Hub weiter. Dieser prüft, ob die betroffene Charge in einem anderen Markt gespeichert ist und leitet die Anfrage entsprechend weiter. Das nationale System des anderen Marktes führt die Prüfung durch. Entsprechend wird das Ergebnis an den EU-Hub zurückgesendet. Dieser wiederum leitet das Ergebnis an das anfragende nationale System und damit an die Apotheke weiter.
Find a contact
Do you have any questions or need help? Don't worry, we are here to help you. Simply use our contact form and we will forward your request to the right contact person, who will get back to you as quickly as possible. Your stress is our concern – let us help you.
contact us